How flow cytometry works
and considerations for the best results
How flow cytometry works and considerations for the best results
Defining flow cytometry and how it is used as a single-cell analysis tool for characterizing biological systems in translational and clinical research
Flow cytometry is a method used for the quantitative and qualitative analysis of single cells in solution. By simultaneously measuring multiple biomarkers on individual cells, it provides detailed characterization of biological systems. Flow cytometry is important to many life sciences fields, including immunology, infectious disease, cancer biology and neurology, in addition to applications for drug discovery and development and precision medicine.
What is flow cytometry
Cytometry is defined as “cell measurement,” so flow cytometry is the measurement of cells flowing through a system. Flow cytometry detects and identifies cell types, biomarkers and immune response, allowing increasingly complex details of cell biology to be understood.
Analysis of heterogeneous cell populations by flow cytometry can be conducted from sample types including blood, bone marrow and solid tissues such as lymph nodes, spleen, mucosal tissues or solid tumors that can be dissociated into single cells.
As flow cytometry has evolved, new variations of the technology have been developed to address limitations with the original approach. These include two options:
Spectral
flow cytometry
A persistent challenge in fluorescence flow cytometry is increasing the number of parameters that can be detected in one experiment. The more parameters used, the more fluorophores needed, and the more complex experiment design becomes. Spectral flow cytometry enables the detection of all emitted wavelengths, followed by spectral unmixing to view every wavelength individually. However, spectral flow cytometry still suffers from difficult panel design, trouble with too many parameters and unmixing issues that hinder accurate analysis.
Mass
cytometry
Mass cytometry captures an even higher number of surface and functional markers simultaneously with lower coefficients of variation compared to flow cytometry. The resulting data is very reliable in large-scale, longitudinal studies, even from rare cell subsets and low abundance intracellular and functional targets. This multiparametric approach facilitates global immune profiling and biomarker discovery that can shed light on new drug candidates and correlates of disease.
Curious when to use which technology for your experiments?
Hear Jennifer Snyder-Cappione explain the differences and the significance of measuring function.
How it works
A typical flow cytometry workflow contains four main steps: Samples must be prepared as a single-cell suspension; cells undergo staining; the stained cells are passed through a cytometer; and the generated data can be analyzed.
Different antibody tags can generate stronger results. Here's how.
Ensuring proper sample prep and configurations for a flow cytometer
Flow cytometry fluidics
The cell suspension first flows through a fluidics system to focus the solution and ensure that only one cell passes through the light sources and detectors at a time. Preparing a single-cell suspension is key to obtaining proper phenotyping data from each cell, maintaining a cell population with high viability and preserving cell surface antigens.
Lasers and optics
Once the cells are focused into a single cell stream, they pass through a series of lasers and optics that deflect light off of the cells. Each cell is analyzed for visible light scatter and one or multiple fluorescence parameters. Light scatter helps distinguish cell types, where forward scatter indicates size and side scatter suggests relative complexity. It is important to match laser and filter configurations of an instrument with the excitation and emission spectra of the chosen fluorophores.
Detectors and filters
Light and fluorescence detection depends on the color and quantity of the lasers used. Detection of different wavelengths requires multiple channels and detectors to capture a mixture of emitted light. Subsequent cytometric analysis distinguishes wavelengths to separate the detected proteins. Filters can also be applied to increase the specificity of detection for chosen wavelengths.
Read about the importance of the number of parameters flow cytometry can measure and what advances are changing how these systems are used for better biomarker discovery.
Things to consider when using fluorescence flow cytometry
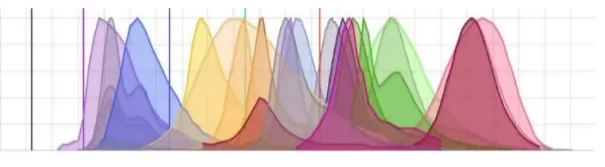
Fluorescence flow cytometry is based on the detection of fluorescent markers conjugated to antibodies, DNA binding dyes, viability dyes and ion indicator dyes, as well as expression of fluorescent proteins. This applies to both conventional and spectral flow cytometry. Since colors primarily fall within the visible spectrum, between wavelengths of about 380–700 nanometers, there is a finite number of fluorophores possible.
Significant planning must be done to balance co-expression, signal overlap and fluorophore brightness, all of which influence the number of parameters that can be measured. Depending on the level of protein expression targeted by each antibody, the fluorophore type must be matched to a protein with an expression level such that no fluorophore will be too bright to cover other signals or so dim as to go undetected.
Distinguishing detected wavelengths
Compensation of fluorescent signals must be included in post-acquisition analysis to correct spectral overlap between different fluorophores. This overlap can lead to signal spillover, where the emission from one fluorophore contaminates the signal of another. Compensation adjustments must be made to correct this, but these are often subjective and can introduce errors and variability into the data. There is no need for compensation in mass cytometry analysis.
Combining phenotype and function
Flow cytometry is a cell biology standard for immunophenotyping and evaluating cell cycle status, monitoring cell death and proliferation and elucidating signaling pathways. Intracellular detection of molecules such as cytokines, chemokines and transcription factors has become increasingly of interest as more is discovered about the complexity of the immune response. Due to the harshness of fixation and permeabilization protocols, care must be taken when choosing antibodies and fluorophores to ensure information from the sample is preserved. These factors can limit conventional and spectral flow cytometry experiments, but is not an issue for the simultaneous detection of surface and functional markers in mass cytometry protocols.
Controls for data visualization
Preparing controls when using fluorescence dyes is a vital step for data validation and to discriminate data from background variation and nonspecific effects, as well as for instrument calibration and differences in sample types. Controls can include known negative and known positive samples, fluorophore controls to ensure gating to the right channels, autofluorescence controls for cells in which autofluorescence can confound signals and antibody binding controls to negate off-target binding. It is important to choose the correct controls for an experiment, depending on which factors are present that can impact results.
High-dimensional analysis with mass cytometry
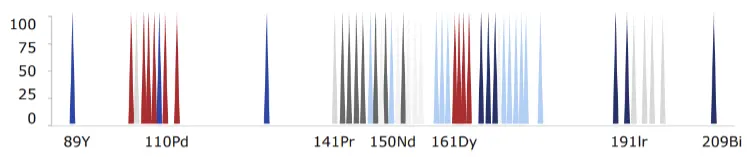
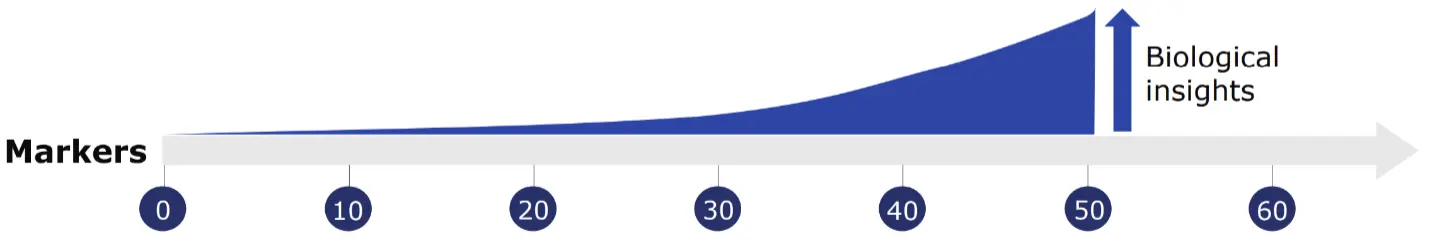
Cytometry by time-of-flight or mass cytometry (on which CyTOF® technology is based) is a pioneering technology developed to overcome many of the challenges experienced with fluorescence flow cytometry, increasing its utility by enabling the simultaneous assessment of a much higher number of parameters. This added capability maximizes the information obtained from each sample and, subsequently, each cell.
Mass cytometry is particularly advantageous because of its ability to precisely and accurately distinguish between reporter molecules. By using atomic mass measurements, mass cytometry avoids autofluorescence signals from cells and tissues, enables fast panel design since all antibodies are used at once and facilitates barcoding to multiplex samples in large-scale acquisitions.
Learn more about mass cytometry and how it works
High-dimensional analysis with mass cytometry



Cytometry by time-of-flight or mass cytometry (on which CyTOF® technology is based) is a pioneering technology developed to overcome many of the challenges experienced with fluorescence flow cytometry, increasing its utility by enabling the simultaneous assessment of a much higher number of parameters. This added capability maximizes the information obtained from each sample and, subsequently, each cell.
Mass cytometry is particularly advantageous because of its ability to precisely and accurately distinguish between reporter molecules. By using atomic mass measurements, mass cytometry avoids autofluorescence signals from cells and tissues, enables fast panel design since all antibodies are used at once and facilitates barcoding to multiplex samples in large-scale acquisitions.
