A tale of two technologies
A head-to-head comparison of mass cytometry and fluorescence cytometry has crucial implications for fighting the inflammation epidemic
Inflammation, the body’s response to illness, injury or other foreign stimuli (such as germs or toxic substances), is a key defense mechanism that allows the immune system to remove harmful attackers. It’s a vital and natural reaction that promotes healing and helps defend against tissue damage.
During the first phase of the inflammatory response, immune cells rush to clear the invader by mounting antigen-specific immune responses. Then, when the immune system senses that the pathogen is no longer a threat, it sends out signals to cease inflammation and cell killing. Finally, the immune system restores tissue homeostasis and stimulates the removal of cellular debris.
However, due to the presence of certain social, psychological, environmental and biological factors, the immune system can fail to resolve its acute inflammatory response. Chronic inflammation, defined as slow, long-term inflammation lasting several months to years, occurs when the body continues to activate inflammatory signals even when there’s no danger. Normally helpful processes end up hurting the body, leading to a breakdown of immune tolerance, with alterations in tissues, organs and cellular physiology, including impaired immune function.
Chronic inflammatory diseases are the most significant cause of death in the world, and the WHO ranks chronic diseases as the greatest threat to human health. Some chronic inflammation-mediated diseases are:
- Diabetes
- Cardiovascular diseases
- Arthritis and joint disease
- Allergies
- COPD
Conditions linked to chronic and/or aberrant inflammation are on the rise globally and often have limited treatment options. Healthcare systems are strained and, in many areas, healthspans – the period of life spent in good health, free from chronic disease and the disabilities of aging – are trending shorter despite our lifespans getting longer.
So how can we combat the growing epidemic of chronic inflammation? Jennifer Snyder-Cappione, PhD, Assistant Professor of Virology, Immunology & Microbiology at the Boston University Boston University Chobanian & Avedisian School of Medicine, wanted to examine that question, delving deeper into how functional profiling can open new paths for targeted therapies.
In an effort to better understand the role of innate T cells in the chronic inflammation found with antiretroviral therapy-suppressed HIV infection, the Snyder-Cappione Lab spent months building a 32-color spectral panel. Spectral flow cytometry resulted in no detectable stimulation-specific signal for IL-10 and IL-13. When the team performed an ELISpot assay to quantify cytokine levels with a different method, results showed that IL-10- and IL-13-producing cells were present at frequencies in PBMC that should have been detected using flow cytometry.
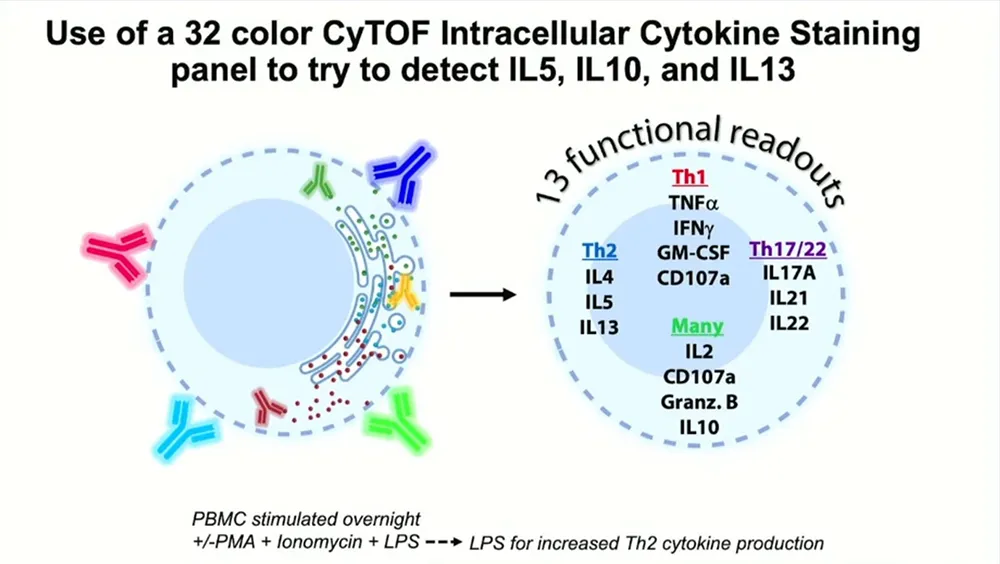
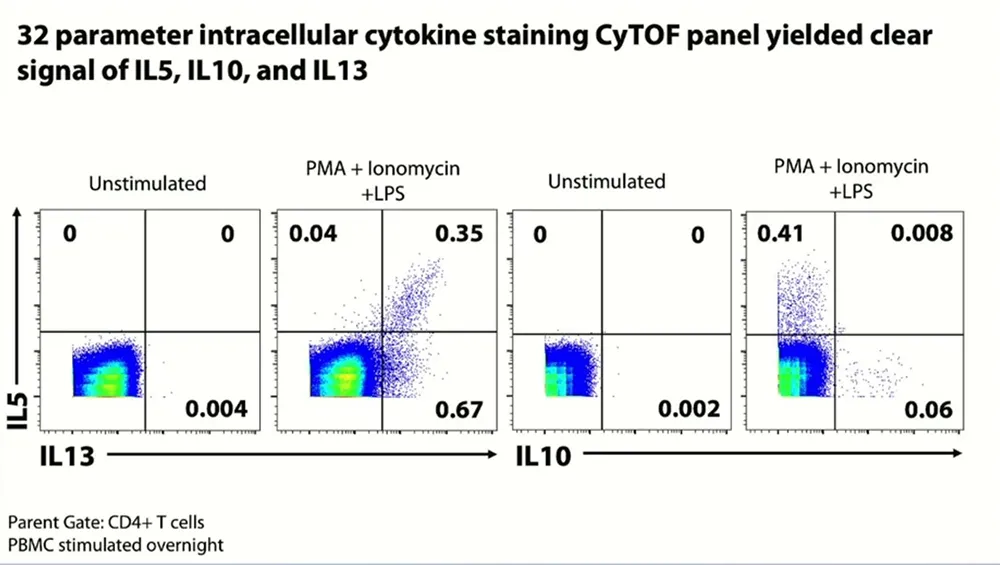
The team were able to quickly pivot to mass cytometry, building a 35-parameter CyTOF intracellular cytokine staining panel to try to detect those same three cytokines. The results were edifying: “Frankly, I was amazed,” Snyder-Cappione says. The CyTOF panel yielded a “superclean” background with “really nice” signal for a variety of cytokines, including not just IFNγ and TNFα but also IL-5, IL-10 and IL-13. Additional opt-SNE analysis, which enables high-quality visualization of megascale cytometry and transcriptomics datasets, revealed T regulatory 1 cells, which simultaneously produce both IFNγ and IL-10, and subsets of Tc2 cells, CD8+ T cells that produce differing combinations of IL-5, IL-10 and IL-13, all achieved uniquely with CyTOF technology.
In order to determine if there was evidence of inherent differences in intracellular target capability between mass and fluorescent cytometry, the Snyder-Cappione Lab wanted to compare the intracellular detection capabilities of the CyTOF XT system against a spectral fluorescent cytometry platform with minimal impact from spectral unmixing/signal spillover. The team developed three small (11–12 parameter) panels with minimal spectral overlap, using identical antibody clones and samples from the same culture wells to detect cytokines, transcription factors and phospho-proteins.
- A 12-parameter intracellular cytokine staining panel was designed to detect IL-5, IL-10 and IL-13. Mass cytometry and fluorescence cytometry showed comparable results for IL-5. However, IL-10 and IL-13 were only cleanly detected using CyTOF technology.
- An 11-parameter panel for intranuclear detection of phosphorylation events was designed to detect pSTAT1, pSTAT3, pSTAT5, p38 and pERK1/2. The team saw a higher resolution of intracellular phosphorylation events using mass cytometry. “The data is overwhelmingly better,” Snyder-Cappione says.
- A 12-parameter panel for intranuclear detection of T cell lineage transcription factors was designed to detect Tbet, TOX, GATA3 and FoxP3. Using CD4+ T cells, better signal was seen for Tbet and TOX with CyTOF technology. Tbet in particular showed a “really dramatic” difference in signal to noise, and twice as many CD8+ T cells came up positive for Tbet as they did in flow cytometry analysis.
The researchers concluded that mass cytometry was “universally better” for measuring cytokine production, phosphorylation events and T cell lineage transcription factors. This could be due to mass cytometry’s mechanics: In fluorescence cytometry, light and emitted signal must pass through many layers of an intact cell, which might result in signal loss or perturbation. Mass cytometry atomizes cells, meaning there is virtually no background signal and no known sources for loss of signal.
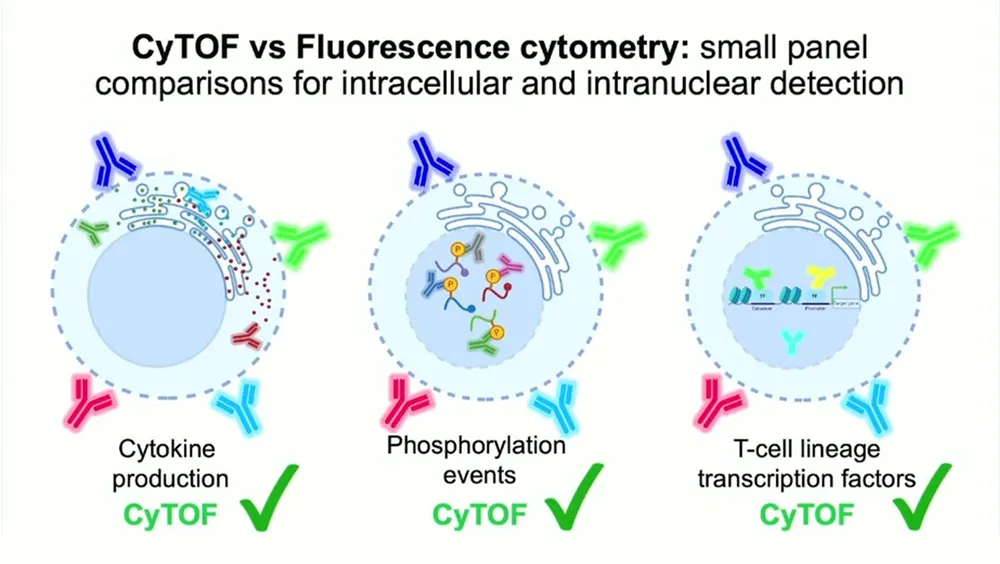
The Snyder-Cappione Lab’s head-to-head comparison of mass cytometry vs. fluorescence cytometry demonstrates why CyTOF technology’s proven sensitivity, dynamic range and resolution are ideal for simultaneously capturing 50-plus targets. CyTOF technology alone enables researchers to obtain functional readouts from critical immune subsets by reliably detecting intracellular markers and signaling proteins, an essential step toward biomarker discovery and improved immunotherapies.
This ongoing study is part of a collaboration between Boston University and Standard BioTools. The statements and opinions shared by Snyder-Cappione are her own and do not necessarily reflect the views of her employer.
